1.2 Million Reports of Injuries After C0VID Vaxxines, VAERS Data Show
By Megan Redshaw
VAERS data released Friday by the Centers for Disease Control and Prevention included a total of 1,247,131 reports of adverse events from all age groups following COVID-19 vaccines, including 27,532 deaths and 224,766 serious injuries between Dec. 14, 2020, and April 22, 2022.
The Centers for Disease Control and Prevention (CDC) today released new data showing a total of 1,247,131 reports of adverse events following COVID-19 vaccines were submitted between Dec. 14, 2020, and April 22, 2022, to the Vaccine Adverse Event Reporting System (VAERS). VAERS is the primary government-funded system for reporting adverse vaccine reactions in the U.S.
The data included a total of 27,532 reports of deaths — an increase of 183 over the previous week — and 224,766 serious injuries, including deaths, during the same time period — up 1,930 compared with the previous week.
Excluding “foreign reports” to VAERS, 810,171 adverse events, including 12,672 deaths and 80,743 serious injuries, were reported in the U.S. between Dec. 14, 2020, and April 22, 2022.
Foreign reports are reports foreign subsidiaries send to U.S. vaccine manufacturers. Under U.S. Food and Drug Administration (FDA) regulations, if a manufacturer is notified of a foreign case report that describes an event that is both serious and does not appear on the product’s labeling, the manufacturer is required to submit the report to VAERS.
Of the 12,672 U.S. deaths reported as of April 22, 16% occurred within 24 hours of vaccination, 20% occurred within 48 hours of vaccination and 59% occurred in people who experienced an onset of symptoms within 48 hours of being vaccinated.
In the U.S., 572 million COVID-19 vaccine doses had been administered as of April 23, including 338 million doses of Pfizer, 215 million doses of Moderna and 19 million doses of Johnson & Johnson (J&J).
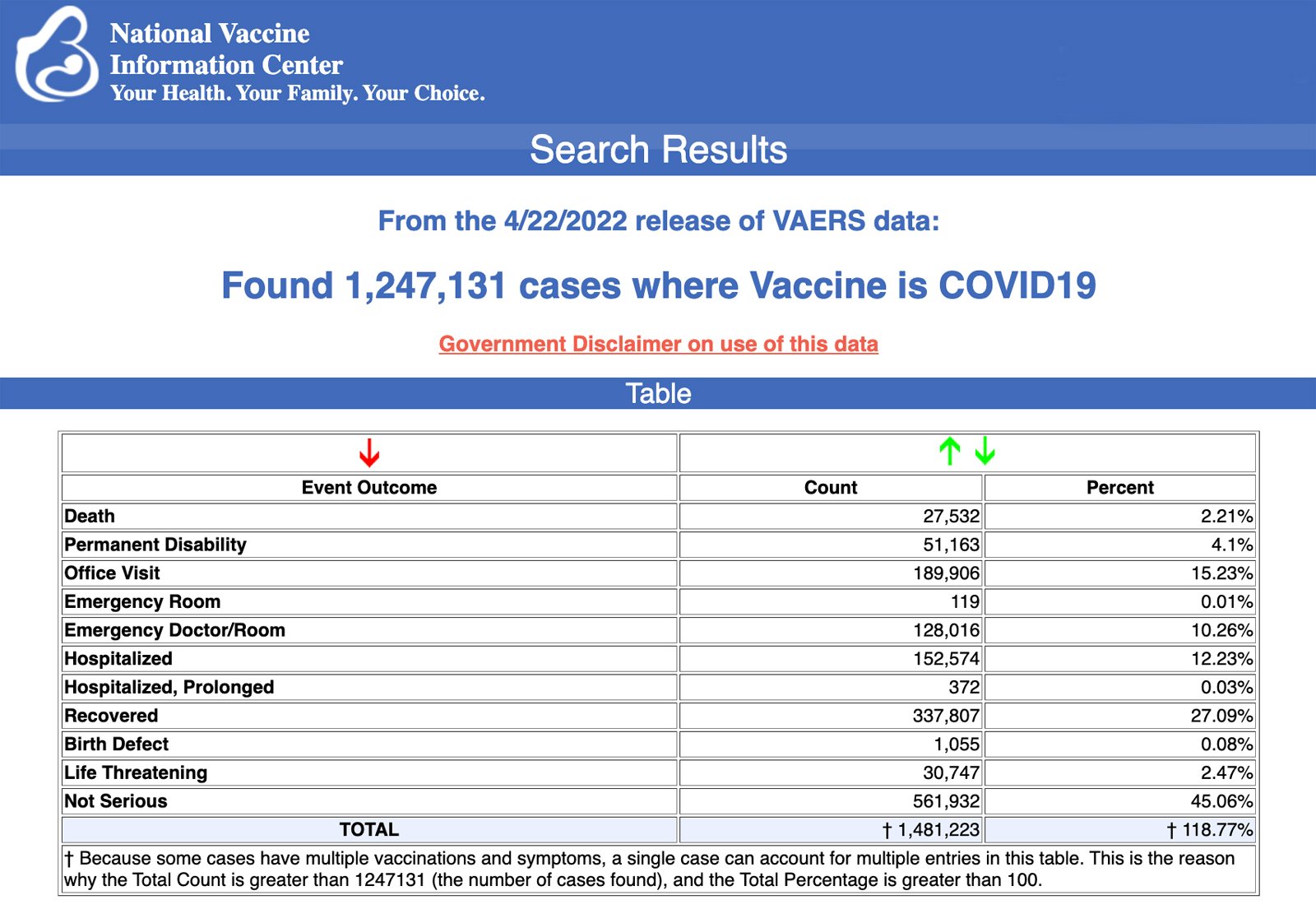
Every Friday, VAERS publishes vaccine injury reports received as of a specified date. Reports submitted to VAERS require further investigation before a causal relationship can be confirmed.
Historically, VAERS has been shown to report only 1% of actual vaccine adverse events.
U.S. VAERS data from Dec. 14, 2020, to April 22, 2022, for 5- to 11-year-olds show:
- 10,348 adverse events, including 256 rated as serious and 5 reported deaths.
- 19 reports of myocarditis and pericarditis (heart inflammation).
The CDC uses a narrowed case definition of “myocarditis,” which excludes cases of cardiac arrest, ischemic strokes and deaths due to heart problems that occur before one has the chance to go to the emergency department.The Defender has noticed over previous weeks that several reports of myocarditis and pericarditis have been removed by the CDC from the VAERS system in this age group. No explanation was provided. - 42 reports of blood clotting disorders.
U.S. VAERS data from Dec. 14, 2020, to April 22, 2022, for 12- to 17-year-olds show:
- 31,455 adverse events, including 1,803 rated as serious and 44 reported deaths.The most recent reported death involves a 14-year-old girl from Tennessee (VAERS I.D. 2238618) who died after receiving her second dose of Pfizer’s COVID-19 vaccine. According to the VAERS report, the girl had a previous history of cancer but was hospitalized 29 days after receiving her second dose of Pfizer with severe COVID-19 and COVID pneumonia. She became “critically ill,” developed respiratory failure and bradycardia and later died.
- 65 reports of anaphylaxis among 12- to 17-year-olds where the reaction was life-threatening, required treatment or resulted in death — with 96% of cases attributed to Pfizer’s vaccine.
- 649 reports of myocarditis and pericarditis — two fewer than last week — with 637 cases attributed to Pfizer’s vaccine.
- 165 reports of blood clotting disorders — 1 fewer than last week — with all cases attributed to Pfizer.
U.S. VAERS data from Dec. 14, 2020, to April 22, 2022, for all age groups combined, show:
- 20% of deaths were related to cardiac disorders.
- 54% of those who died were male, 41% were female and the remaining death reports did not include the gender of the deceased.
- The average age of death was 73.
- As of April 22, 5,460 pregnant women reported adverse events related to COVID-19 vaccines, including 1,709 reports of miscarriage or premature birth.
- Of the 3,630 cases of Bell’s Palsy reported — three fewer than last week — 51% were attributed to Pfizer vaccinations, 40% to Moderna and 8% to J&J.
- 870 reports of Guillain-Barré syndrome, with 42% of cases attributed to Pfizer, 30% to Moderna and 28% to J&J.
- 2,343 reports of anaphylaxis — 12 fewer reports than last week — where the reaction was life-threatening, required treatment or resulted in death.
- 1,678 reports of myocardial infarction.
- 13,826 reports of blood-clotting disorders in the U.S. Of those, 6,199 reports were attributed to Pfizer, 4,925 reports to Moderna and 2,661 reports to J&J.
- 4,152 cases of myocarditis and pericarditis with 2,544 cases attributed to Pfizer’s, 1,415 cases to Moderna’s and 181 cases to J&J’s COVID-19 vaccine.
FDA to meet in June on COVID-19 vaccines for babies, toddlers
The FDA will meet in June — either June 8, 21 or 22 — to discuss COVID-19 vaccines for children under age 6. The agency’s vaccine advisory committee during its June meeting will also discuss Novavax’s request for Emergency Use Authorization (EUA) of its COVID-19 vaccine for adults.
Moderna on Thursday asked the FDA to authorize its COVID-19 vaccine for emergency use for children ages 6 months to 6 years.
The company conducted separate trials for two versions of the vaccine, one for infants and toddlers 6 months to 2 years, and one for children 2 to 6 years old.
The company claimed data showed “a robust neutralizing antibody response” and “a favorable safety profile.” But experts say Moderna is not providing the data needed to calculate the risk-benefit of its COVID vaccine.
Moderna’s KidCOVE study cited in Thursday’s press release shows the Moderna shot failed to meet the FDA’s minimum efficacy requirements for EUA in the 2- to under-6 age group, and barely surpassed the agency’s 50% efficacy requirement in the 6-month to 2-year age group after the vaccine maker changed its analysis of the study to meet the threshold.
In the younger age group, Moderna said the effectiveness of its vaccine was 51%. In the older age group, vaccine efficacy was only 37% — substantially lower than the FDA’s requirement. These are different efficacy numbers than those the company reported last month.
Pfizer is expected to file its application in May for a three-shot vaccination using smaller individual doses for children under 5.
Lawmakers push FDA on COVID-19 shots for youngest age groups
Moderna’s announcement followed just days after the House Select Subcommittee on Coronavirus Crisis asked the FDA for a status update on COVID-19 vaccines for children under 5 over concerns “millions of young children still remain unprotected because no vaccine has yet been authorized” for this age group.
A top FDA official on Tuesday told The New York Times the agency has not cleared a COVID-19 vaccine for the youngest age group because Pfizer and Moderna have not finished their applications for authorization.
The agency said last week it is considering holding off on reviewing Moderna’s request to authorize its COVID-19 vaccine for children under 5 until it has data from Pfizer and BioNTech on their vaccine for children, pushing the earliest possible authorization of a vaccine from May to June.
The FDA said it would be simpler and less confusing to simultaneously authorize and promote two vaccines to the public, rather than green-lighting one on a faster timetable and the other down the road.
Agency officials were worried about authorizing Moderna’s vaccine only to find out just a few weeks later that Pfizer’s offered better protection.
Pfizer requests EUA for booster dose for 5- to 11-year-olds
Pfizer and BioNTech on Tuesday announced they applied for EUA of a COVID-19 booster dose for children ages 5 to 11. In a press release, Pfizer cited data from its Phase 2/3 trial that claimed a third dose produced a “strong immune response” in the younger age group when administered six months after the second dose.
The data was based on a small study involving only 140 children 5 through 11 years old who received a booster dose six months after the second dose of Pfizer-BioNTech’s COVID vaccine as part of the primary series.
Pfizer said 30 children who participated in the study revealed a 36-fold increase in virus-fighting antibodies — levels high enough to fight the Omicron variant, which is currently not the dominant variant in the U.S.
Experts told The Defender the “clinical trial used to support the notion of a COVID-19 booster for 5- to 11-year-olds is entirely inadequate to make any such recommendation.”
Denmark suspends COVID-19 vaccine campaign
Denmark on Tuesday became the first country to suspend its national COVID-19 vaccine campaign after health officials said the pandemic is under control there.
Bolette Soborg, director of the Danish Health Authority’s department of infectious diseases, said Denmark is “winding down” the mass vaccination program, and invitations for vaccinations would no longer be issued after May 15.
Public health authorities cited several factors contributing to the decision to end the national vaccination campaign. These include a decline in the number of newly reported infections, stabilized hospitalization rates and an overall high level of vaccination.
Denmark plans to reopen the vaccination program in the fall, which will be preceded by a thorough professional assessment of who and when to vaccinate, and with which vaccines.
The decision comes just a few months after Denmark eliminated all COVID-19-related restrictions, becoming the first European Union member state to do so.
Children’s Health Defense asks anyone who has experienced an adverse reaction, to any vaccine, to file a report following these three steps.

