Whoops! The Together Trial Actually Showed That Ivermectin Worked
By Steve Kirsch
Even the author admitted it. The media and medical establishment did not read the study carefully. They seize on anything that supports the narrative and fail to look at the study critically.
I just got off the phone with Pierre Kory. He just got back into the country and on Monday, April 4, he will be writing an article on his Substack on the ivermectin arm of the Together Trial. Let’s just say Pierre is not a happy camper. I haven’t seen him this upset ever.
Here is what Peter McCullough wrote: “Dose too small and for too few days too late in course of illness. Despite all of these deficiencies, this small underpowered trial still showed a signal of benefit and presumed acceptable safety. Another paper supporting our use of IVM.”
I agree. Here’s my take on this.
Ivermectin works and this study didn’t prove otherwise
The most important thing is this: The evidence, including this study, consistently shows that ivermectin works against COVID. In one group, as I will explain below (and I’m sure Pierre will explain in more detail), there was a 50% reduction in hospitalization. That’s big.
However, the media (including Alex Berenson) don’t want to look like they were wrong so they simply misinterpret the study results and hope you won’t read the study yourself.
What the study actually showed
Disease, timing, dose, duration, and statistical power are all important to talk about whenever you talk about the results of any drug trial. There are other secondary factors regarding gaming, tampering, quality control, etc. that are important and are always overlooked. Let’s just address the biggest factors.
What the study showed is this:
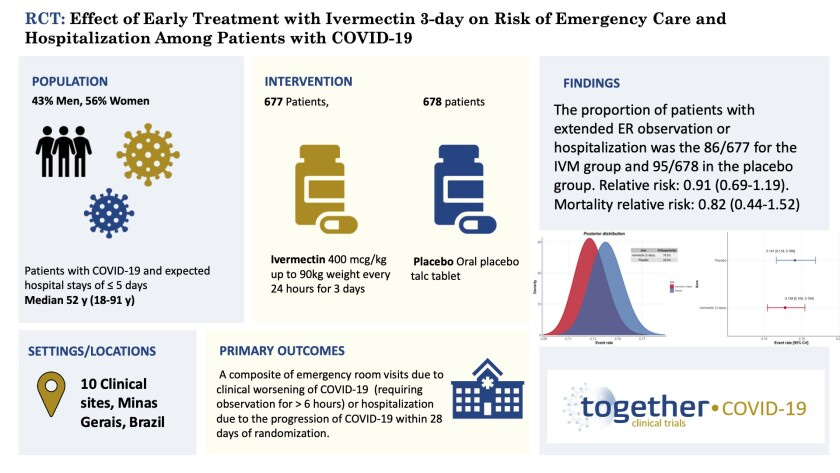
For the P.1 variant of the virus, giving a low dose of ivermectin (0.4mg/kg) for just 3 days starting up to 8 days days after first symptoms as a monotherapy (i.e., with no other drugs) results in a small effect that requires more patients in your study than you originally thought to prove it is statistically significant.
That doesn’t mean the drug didn’t work. It just means it was underpowered for the variant it was given against as a monotherapy for just 3 days.
It’s pretty clear that had the study enrolled more patients, ivermectin would have “worked” and the results would have been statistically significant. The author admits this.
Here are some key points everyone needs to know about the trial
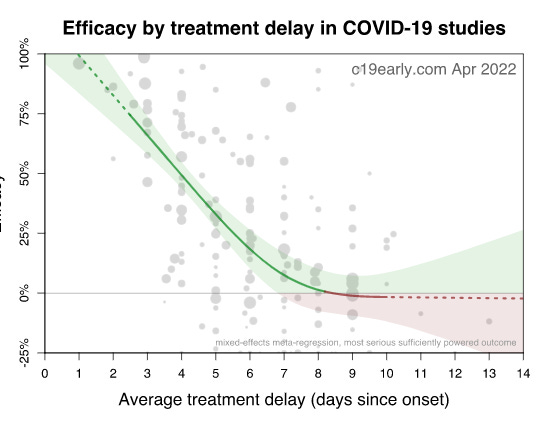
- Patients waited up to 8 days after symptoms before starting treatment; the average was 3-4 days after symptoms. Basically, if you wait 8 days before treatment, your drug will fail:
- For the P.1 variant (aka gamma), it should be given it at 0.6mg/kg for patients not responding to the lower dose, not keeping it fixed at 0.4mg/kg
- You need to give it for as long as symptoms persist (and not stop after 3 days). This is especially important for the P.1 variant that was common at the time of the trial.
- Ivermectin is most effective in COMBINATION with other drugs (such as zinc), not alone. Nobody gives it alone.
- It’s really important to make sure you are running the placebo and ivermectin arms at the same time since the variants can change over time. Gamma was prevalent at ivermectin time which was a significant disadvantage and was not present for the placebo group. This puts the drug at a significant disadvantage.
- It’s important to make sure the people in the placebo arm aren’t taking the study drug by listing it as an exclusion. I’ve heard this wasn’t officially listed, but Ed says everyone was asked about this.
- 450 people fled the trial in the control group and it was never mentioned (the IVM cohort had just 50 people drop out). This means the control people likely dropped from the trial when they realized they weren’t getting the drug.
- Why did it take 7 months to get the study published in the NEJM in a pandemic?
- In my opinion, the most interesting thing in the whole study is that people who have analyzed the data for the “unknown” group (those not in the 0 to 3 or 4 to 7 day before starting treatment) found that ivermectin had a 50% reduction in the primary endpoint (hospitalization). This is a huge benefit that was not noted in the NEJM paper. I wonder why? Pierre I’m sure will be talking about this.
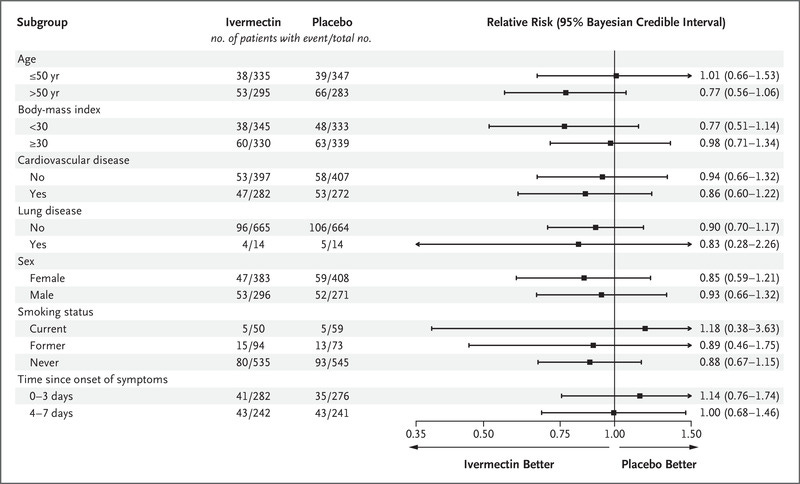
- If ivermectin doesn’t work, why would it be superior in 12 of the 15 categories they looked at? Also, it’s really bizarre that those who took it earlier fared worse than those who took it later (last two lines):
That’s a quick summary of the learnings. The same learnings are true with other drugs like fluvoxamine. However, the medical community is very slow to learn these lessons that timing, dosing, etc. matter. For example, David Wiseman found that HCQ actually worked and it was statistically significant if you analyzed the data correctly and took into account the FedEx shipping delay. Wiseman tried to get his results published, but the journals didn’t want to upset the narrative and rejected his work. I’ve known David since the start of the pandemic and I can tell you he is meticulous. I’ve seen top scientists duck and run for cover when Wiseman asks to see their data.
The key points are:
- Be careful not to generalize the result of a trial. A given trial means that drug X given with a delay Y and at a dose Z and duration A and…. is not statistically significant if there are only B people in the trial. Changing one or more of these variables can turn a “not statistically significant result” into something that is statistically significant with a big effect size. The press should never say, “HCQ doesn’t work for COVID.” They should say, “If you give HCQ at dose X for duration Y on Z hospitalized patients, there was not a statistically significant effect.” When you give it early in the appropriate dose and duration and in combination with other drugs, it works great as George Fareed and Bryan Tyson (among many others) will attest to.
- Timing, dosing, duration, and combination of drugs all matter. Timing is the most important thing. If you delay for too long, a miracle drug can turn into a dud.
The study also PROVED that the NEJM, mainstream medical community, and the press are incapable of objectively interpreting the results of a study.
It’s important you all know that.
In today’s world, evaluation of studies is driven by agreement with the mainstream narrative, not by a critical look at what the study actually shows. If it agrees with the narrative, the study doesn’t have to be evaluated for flaws.
That is not how science is supposed to work.
Here is what study senior author Ed Mills wrote in a private email to Marc Rendell (used with Ed’s permission)
The bold is mine. This is key: had the ivermectin study enrolled more people, it would have been a statistically significant benefit.
Did you see that admission in any story in the mainstream media? Did you see that admission from anyone in the mainstream medical community? Of course you didn’t! These people don’t look at these studies carefully. They jump to conclusions based on their biases. As a result, a promising drug gets discarded and lives are lost. This is how it works. This is why the mainstream medical community needs a major overhaul that they can all be so wrong.
I know a doctor in Canada who is being forced by his medical board (the College of Physicians and Surgeons of Ontario) not to prescribe ivermectin. This is insane. In my mind, this is criminal. Do you know what their motto is? TRUSTED DOCTORS PROVIDING GREAT CARE. I’m not kidding. You can’t make this stuff up! You can bet a substack is coming about this where I will reveal the letter they sent this doctor as soon as they force the doctor to sign it (or else they take away his license).
Here’s the message from Ed Mills to Marc Rendell on April 3, 2022:
Hi Marc
I hope you are well. Thank you for your email. You have been the only person courteous enough to ask the questions.
I don’t understand the psychology of the ivermectin advocates. They fail to see the positive in this study and just focus on it not being overwhelmingly positive. I actually think it is quite positive.
I presented this a couple weeks ago at the NIH Collaboratory Rounds and, if they listened, I advocate that actually, there is a clear signal that IVM works in COVID patients, just that our study didn’t achieve significance. In particular, there was a 17% reduction in hospitalizations that would be significant if more patients were added. I really don’t view our study as negative and, also in that talk, you will hear me retract previous statements where I had been previously negative. I think if we had continued randomizing a few hundred more patients, it would have likely been significant.
I can answer a few of the questions below today and others will take until Monday or Tuesday when I chat with the statistician.
1) All patients were asked about their previous ivermectin use and excluded if they reported ever using it for COVID. Patients did not choose which intervention they received, but could be blocked from receiving one if they previously had taken it. This is all in the protocol.
2) At the time we did our study, IVM was not particularly popular for use in Minas Gerais. Even if some patients did access IVM, the fact that it is blinded should still maintain balance.
3) These are not discrepant. There are several approaches one can use for this analysis and we wanted to be as data driven as possible.
These are the numbers for where the complete data for that particular variable was not missing. If you use the intention to treat approach, you get fairly identical results, as you can see in the attached slide.
4) Per-protocol in this analysis was only the placebo patients taking the exact matching placebo (3 day matching placebo to the active IVM). This was the journal’s insistence and not ours. Fig 1 is a typo and will be fixed.
5) I don’t understand where they would get this idea. All bottles were labeled nearly identically and no one knew their contents at the point of randomization. Only the randomizing pharmacist was aware of the allocation.
6) I will check on this, but if memory serves me correctly, it is COVID deaths in table 3 and all-cause deaths in Grade 5. This should have been labeled COVID deaths in table 3. We will have that inserted.
7) We are providing all data sets to ICODA. Applicants can then propose an analysis to ICODA and, if they approve it, can get access to the data. It’s not a minor issue to prepare CDISC data sets and we have been swamped with getting the FDA EUA submission on lambda such that our statisticians are occupied with that. The data sets will be available soon.
I don’t know much about the ACTIV-6 study. All that I know is that they discontinued randomizing to the 3 day dosing at the beginning of March. They say they will release their initial findings in May. I have no idea why it is taking them a while to do it, but I can understand that there are always data cleaning issues that are time consuming. I’m not sure about ACTIV 6, but ACTIV 2 was using IQVIA for its data collection and patient recruitment, so should have been a lot faster than it currently is. I know that the University of Minnesota study has concluded but I also don’t know anything about the study. I understand their metformin and also fluvoxamine evaluations were positive.
Best wishes
Ed
My personal feelings about Ed Mills and Pierre Kory
I’ve known Ed since 2020. He’s always been extremely nice to me and has never ducked any questions.
When he started the ivermectin trial, he told me he was skeptical that it worked, but he was willing to go with what the data shows. What’s clear from his email is that at the end, he was convinced it was effective. That’s significant. A dishonest scientist wouldn’t say that.
I’ve known Pierre for about the same amount of time as Ed and I have great respect for him as a man of high integrity and enormous courage to challenge the mainstream narrative. He’s amazing.
So I have a lot of respect for both Ed and Pierre and I’m friends with both.
I encouraged Pierre to call Ed on the phone to talk about his objections and see if they could be resolved. That didn’t happen and that’s unfortunate. I’m sure Ed would have happily taken the call.
We are all in this together and it’s important that we work together to resolve differences of opinion. I am continuing to encourage Pierre to give Ed a call and have a friendly discussion.
Not meeting to resolve differences of opinion is unproductive
In all cases to date, the blue-pill “experts” in the vaccine have steadfastly refused to engage with any of us to resolve our differences. We have reached out countless times, even with offers to “name your price,” to have our questions answered, but to no avail. Nobody wants to explain to us how we got it wrong. Not even for a million dollars. This is astonishing.
We made the same offer to people who claim that masks protect people from getting or spreading SARS-CoV-2. Everyone we contacted refused to defend their position except for just one person: Yale economics Professor Jason Abaluck. Professor Abaluck was the senior author of the Bangladesh mask study. He said he would engage us, but restricted our sessions to one person at a time. We had our first discussion (2 hour recorded zoom call) on April 3, 2022. I’ll be writing a Substack on this tomorrow when the recording is released.
The important lesson is this: when people do agree to come together, differences get resolved. When people refuse to meet to resolve their differences, the differences don’t get resolved. Such a novel concept!
Hence, it is completely unproductive for people who are pro-vaccine to continue to stonewall answering our questions. If they have nothing to hide, why aren’t they willing to meet with us?
What do you think?